TRIAL
Space Shield RCT: A Space-Expanding Shield in Decompressive Hemicraniectomy for Stroke – an International Multicenter Randomized-Controlled Phase III Trial
Background & Rationale
A malignant cerebral infarction causes cytotoxic edema in the ischemic brain parenchyma eventually resulting in a potentially life-threatening increase of the intracranial pressure (ICP). Standard of care is a surgical removal of a part of the skull above the ischemic brain, the so-called decompressive hemicraniectomy (DCE).
DCE allows the brain to expand and has been shown to improve the survival in randomized-controlled trials. After detumescence of the brain parenchyma the patients undergo a second surgery after several months with implantation of either their own preserved bone flap or a bone flap substitute (cranioplasty (CP)).
Despite its proven life-saving benefit, this strategy of DCE followed by CP has several risks. Brain herniation through the craniectomy may cause axonal shearing injuries, as well as infarction and hemorrhage at the craniotomy edges. Swelling of the temporal muscle may compress the temporal lobe, leading to herniation. The lack of shielding by the skull exposes the brain parenchyma to injuries and to the atmospheric pressure. The latter, as well as altered cerebral blood flow and changes in the circulation of the cerebrospinal fluid may lead to various neurological deficits subsumed under the syndrome of the trephined. Further, subsequent CP again carries additional surgical risks, such as infection, bone resorption, injury of brain parenchyma and hemorrhages which could be prevented by shifting to a single stage surgery.
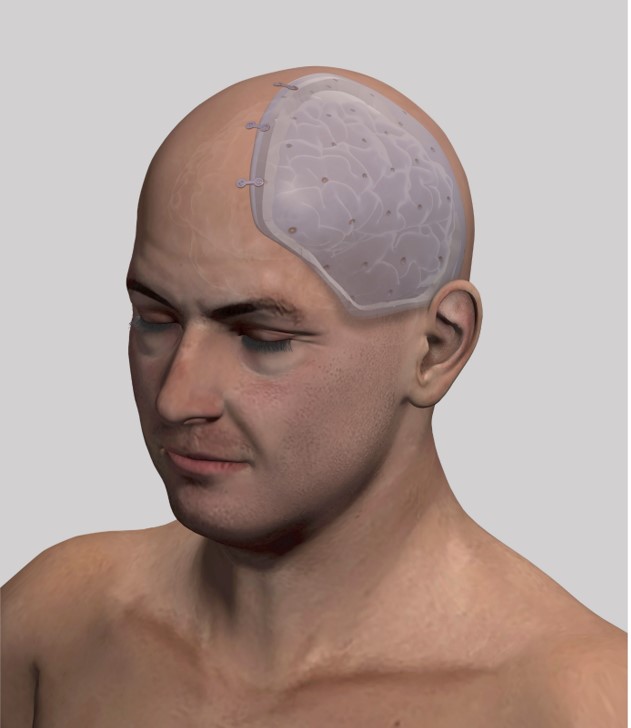
As we showed in previous trials 1,2, many of the above-mentioned risks may be prevented using an intraoperatively molded space-expanding protective shield which is implanted and fixed directly after having performed (a so far customary) DCE. This shield allows the brain to expand while continuing to provide protection.
The proposed trial investigates whether the single-stage strategy of implanting a space-expanding shield represents a viable alternative to the standard DCE followed by CP.
1. Montalbetti M, Lörcher S, Nowacki A, Häni L, Z’Graggen WJ, Raabe A, Schucht P. How much space is needed for decompressive surgery in malignant middle cerebral artery infarction: Enabling single-stage surgery. Brain Spine. 2023 Apr 4;3:101730. doi: 10.1016/j.bas.2023.101730. PMID: 37383456; PMCID: PMC10293220.
2. Schucht P, Nowacki A, Osmanagic A, Murek M, Z’Graggen WJ, Montalbetti M, Beck J, Stieglitz L, Raabe A. Space-expanding flap in decompressive hemicraniectomy for stroke. J Neurosurg. 2022 Jul 22;138(2):382-389. doi: 10.3171/2022.5.JNS22381. PMID: 35901672.

Objective
Despite its proven life-saving benefits, the strategy of DCE followed by CP carries several risks, among others:
- Exposure of the brain parenchyma to atmospheric pressure and trauma
- Various neurological deficits subsumed under the syndrome of the trephined
- Complications of cranioplasty
As shown in a previous prospective cohort study the above-mentioned risks may be prevented using an intraoperatively molded space-expanding protective shield, which is implanted and fixed directly after having performed (a so far customary) DCE. This shield allows the brain to swell while still providing protection.
The here proposed trial investigates whether the single-stage strategy of implanting a space-expanding shield represents a viable alternative to the standard DCE followed by CP.
Study design
The study is designed as an international multicenter randomized-controlled trial
Main inclusion criteria
Malignant infarction of the middle cerebral artery
Main exclusion criteria
Hyperacute need for DCE due to rapid neurological decline
Study Intervention
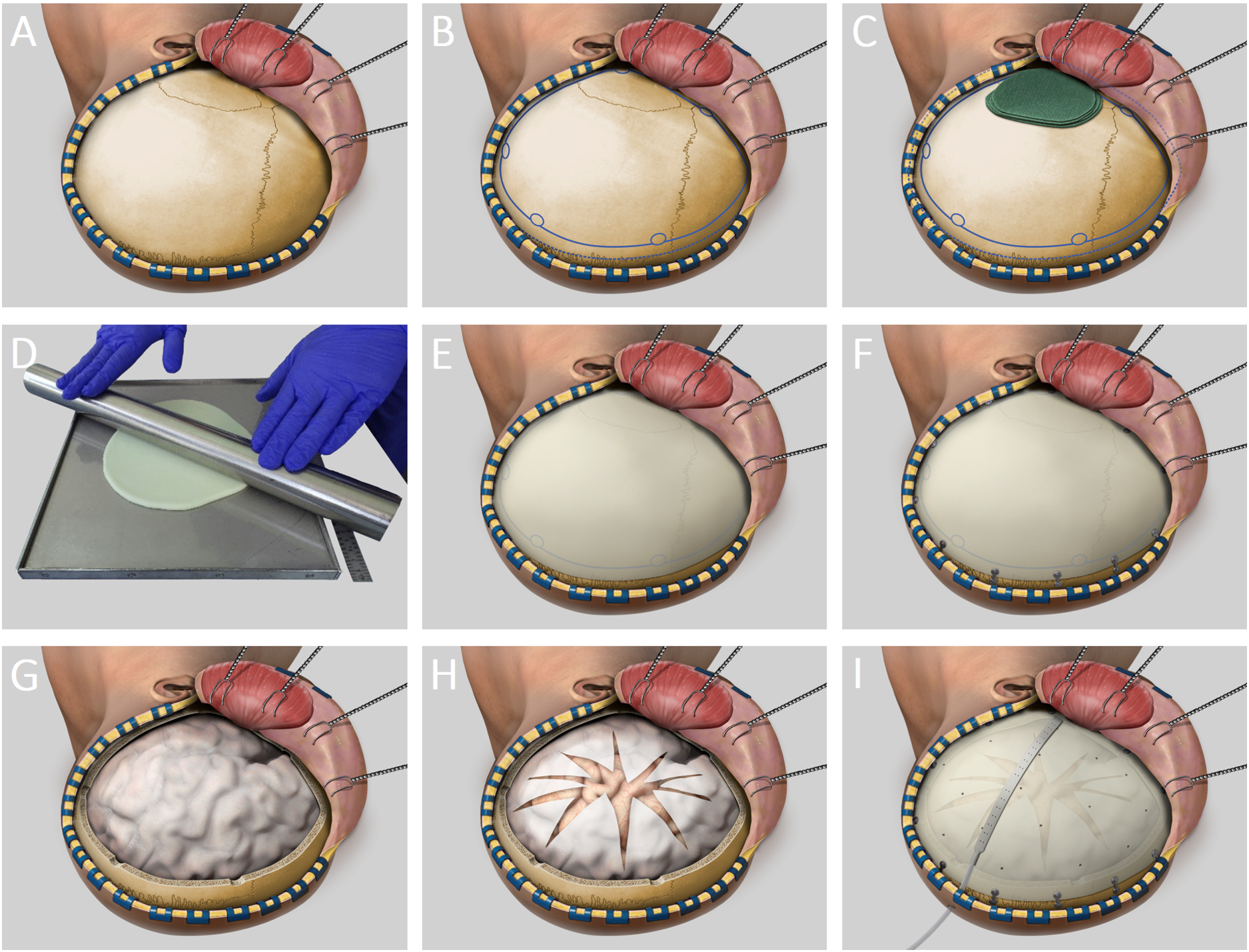
The study intervention is the following surgical procedure: The patients are under general anesthesia and in a semi-lateral position with their head turned laterally. The head is shaved, disinfected, and draped. The skin is incised in the shape of a large question mark from the zygoma to the forehead. Then the temporal muscle is incised and dislocated anteriorly until the zygomatic process can be seen.
After exposure of the cranium and prior to hemicraniectomy, a 3-5 mm flap of CE-certified bone cement made of PMMA (FDA PMA No. P810020, 1984) such as Palacos ® is made using a mold (FlapMold FM3-C40, UID (01)07649998208017(11)241108(21)0001 to (21)0030) made of stainless steel and shaped onto the exposed cranium overlapping the planned craniectomy by 1 cm thus forming the so-called “space-expanding shield”.
Once hardened, the shield is fixed with standard titanium plates and screws to ensure correct positioning. Then the screws on the side of the skull are removed. The space shield is removed and the DCE is performed in the customary manner including opening of the dura to allow brain expansion. The space-expanding shield – with small holes drilled in it – is then fixed in place with low-profile titanium plates. The temporal muscle is readapted over the shield and sutured. Finally, the skin is readjusted and sutured in layers.

Surgical Method with a Space Shield in 9 Steps
Phase 1, exposure (A – C):
Skin incision – Dislocation of the galeal flap – Incision of the temporal muscle – Paddies positioned onto temporal lobe
Phase 2, molding (D – F):
Molding of Palacos® (= bone cement) into a shield of 3-5mm thickness – Fitting of the shield onto the exposed cranium
Phase 3, craniotomy (G – H):
Customary DCE using multiple burr holes and the craniotome – Opening of the dura
Phase 4, implantation (I):
Fixation of the space-expanding shield
Phase 5, closure (I):
Insertion of a drain – Readaptation of muscle and galea flap
Surgical Method in the Control Group
Surgery in the control cohort firstly consists of the above steps 1, 3 and 5 (exposure, craniotomy, closure) while omitting steps 2 and 4, molding and implantation) – a standard DCE.
After detumescence of the brain parenchyma, the patients in the control group will receive a cranioplasty – a surgical repair of the bone defect. The standard-of-care for this procedure is the implantation of a patient-specific implant made of bone cement (Palacos® or other comparable materials). Timing of cranioplasty depends on clinical and radiological results (usually after about 3 months).